Discovery of new skeletal tissue advances regenerative medicine potential
A research team headed by the University of California, Irvine has identified a fresh type of bone tissue that holds significant possibilities for furthering regenerative medicine and tissue engineering advancements.
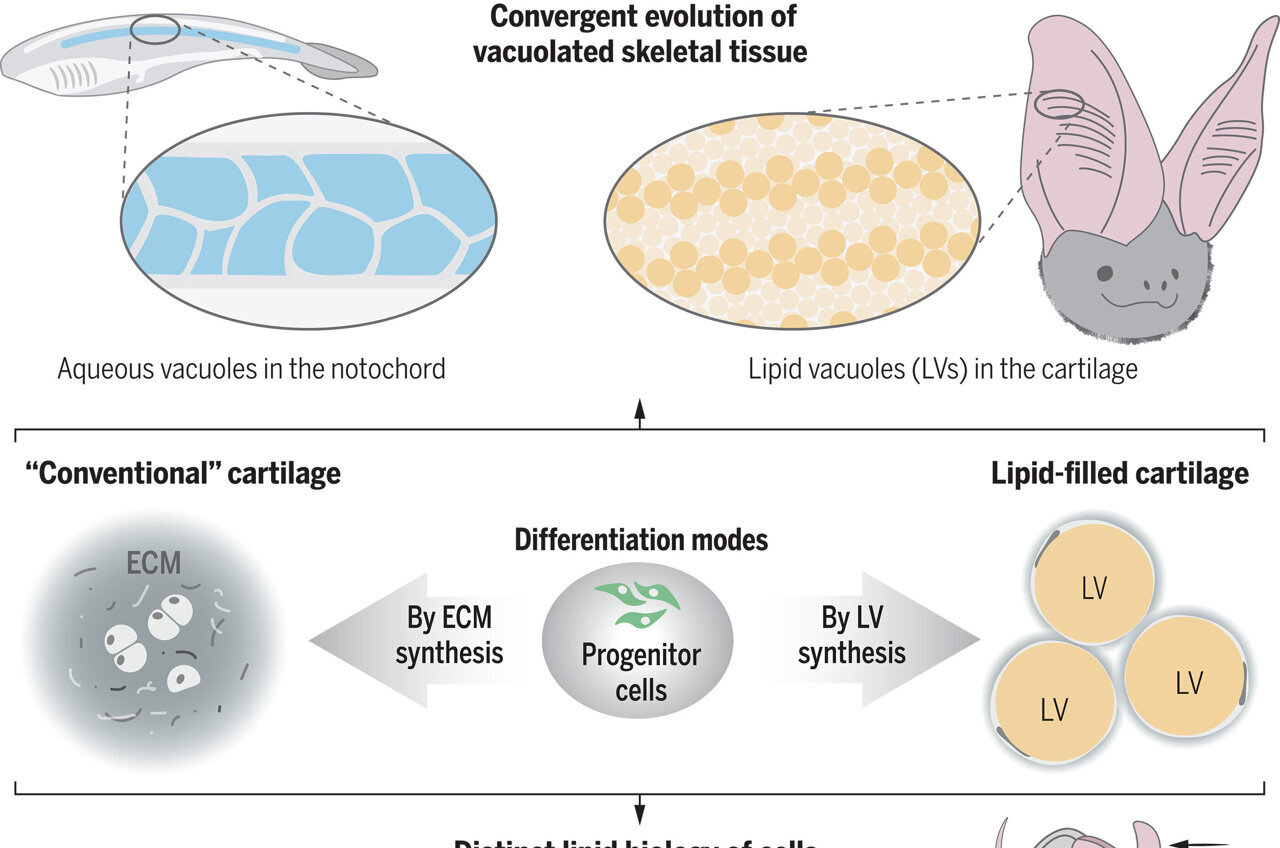
Most cartilage relies on an external layer outside the cells for support, but a unique kind is found in the ears, nose, and throat of mammals, which is packed with cells filled with fat that provide super-strong, internal support, allowing the tissue to remain soft and springy, similar to bubble wrap packing material.
Lipocartilage cells generate and sustain their own fat reserves, with their size remaining consistent. Unusually, unlike typical fat cells, lipochondrocytes do not fluctuate in size in response to the availability of food.
"Lipocartilage's resilience and stability offer a flexible, stretchy quality that's well-suited for body parts like earlobes or the tip of the nose, making it suitable for exciting advances in regenerative medicine and tissue engineering, particularly for repairing facial defects or injuries," said Maksim Plikus, a UC Irvine professor of developmental and cell biology.
At present, cartilage reconstruction usually calls for taking tissue from the patient's rib—a painful and invasive process. In the future, patient-specific cartilage cells called lipochondrocytes could be obtained from stem cells, purified and used to create living cartilage tailored to a person's individual needs. With the aid of 3D printing, these engineered tissues could be shaped to fit exactly, offering new solutions for addressing birth defects, trauma, and various cartilage disorders.
Dr. Franz Leydig first discovered lipochondrocytes in 1854, and he observed that the cartilage of rat ears contains fat droplets, a discovery that had been largely overlooked until now. With the advanced tools of modern biochemistry and imaging techniques, researchers at UC Irvine have thoroughly investigated the molecular biology, metabolism, and structural role of lipochondrocytes in skeletal tissues.
They also discovered the genetic process that prevents certain enzymes from breaking down fats and limits the absorption of new fat molecules, effectively keeping the fat stores of lipochondrocytes locked in place. When these fat cells are depleted, the lipocartilage becomes hard and prone to breaking, which emphasizes the role of fat-filled cells in giving the tissue its balance of toughness and flexibility.
The team also observed that in some mammals like bats, mast cells assemble into complex forms, such as parallel ridges on their large ears, which could improve hearing by adjusting sound waves.
"Lipocartilage's distinct lipid biology shakes up traditional thinking in biomechanics and creates a multitude of new avenues for research," stated the lead author of the study, Raul Ramos, a postdoctoral researcher in the Developmental and Regenerative Biology laboratory run by Dr. Plikus.
Future directions include understanding how lipochondrocytes maintain their stability and how their form and function are controlled by specific molecular programs. Our research has also shed light on the mechanisms of cellular aging. Our findings show that lipids have many uses beyond their role in metabolism and open up new possibilities for using them in tissue engineering and medicine.
The team consisted of healthcare professionals and academics from the US, Australia, Belarus, Denmark, Germany, Japan, South Korea, and Singapore, along with staff from the Serrano Animal & Bird Hospital in Lake Forest and the Santa Ana Zoo.
DOI: 10.1126/science.ads9960
There is no text provided to paraphrase. Please provide the text and I will paraphrase it in United States English language.
Stay current with the latest tech news and scientific breakthroughs.
Post a Comment